The introduction of checkpoint inhibitors in clinical oncology has transformed the way we treat cancer patients. Currently, observed sustained clinical responses have given patients new hope. And yet, these treatments don’t provoke the exact immune response in every patient. The big question is of course, ‘why?’. We asked Dr. Monika Baehner to shed some light on how AI-powered tissue analysis is helping change precision oncology to treat patients better with targeted therapies.
What are the current challenges in immune oncology?
Immunotherapies have incredible potential on a wider scale; but today, unfortunately, they only help a subset of patients. In this quickly evolving field, we acknowledge that the nature of cancer’s complexity requires even more innovative treatment approaches. For example, patients now have access to 5 PD-1/PD-L1 targeting antibodies on the market. And still, even for most approved indications, these drugs are only administered as a monotherapy.
Remarkably, there are over 2,000 ongoing clinical trials that combine checkpoint inhibitors with a whole plethora of interventions, from chemotherapy to targeted therapies and immune targeting drugs. Nonetheless, with all these treatment options, precision medicine is crucial for the oncologist to direct the right patient to the right clinical trial. This, of course, increases the survival outcome but also reduces costs and prevents patient risk from the side effects of ineffective treatments.
How do phenotypes affect clinical trial analysis to deliver precision oncology?
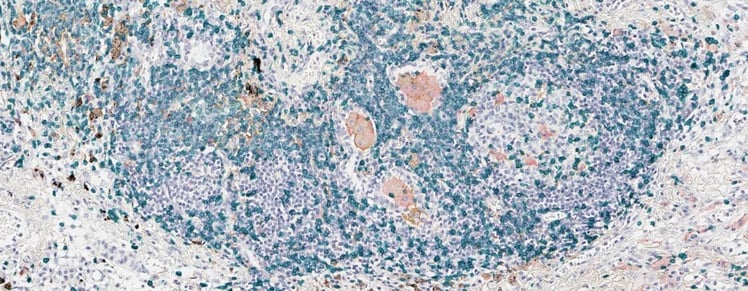
When it comes to delivering the right treatment to the patient, we need to have a deeper understanding of disease biology and its underlying immune mechanisms. In this way, a cancer-relevant phenotype plays out at the tissue level where cancer biology shapes the individual architecture of a tumor and its microenvironment. Therefore, profiling the immune status becomes a critical step to analyze a patient’s tumor.
Visualized by immunohistochemistry (IHC), the information is reflected in spatially resolved biomarker expression levels of both the immune and tumor cells within the context of tissue morphology. In this way, we are able to apply a big data approach to capture and quantify the tissue marker information using advanced image analysis technologies. Depending on the specific scope of the project, we can combine a set of AI technologies, from classical machine learning to deep learning, to deliver the most robust biological insights to expedite drug development.
How does technology like AI for tissue analysis tie into WIN Symposium's theme?
The theme this year for WIN Consortium 2019 in Paris is WINnOvation & Global Deployment of Precision Oncology. WIN has always maintained its commitment to understanding the underlying biology of cancer to better diagnose as well as to choose the right treatment. We know now that genomic analysis at the DNA level is incredibly important, but simply not enough. WIN has already taken the next step to understanding gene expression data at the RNA level. So, it makes sense that the logical third step would be to uncover and use the information on the protein and cellular level revealed within the tissue. Especially in immune-oncology, it is important that we understand the distribution and activation status of the immune cells fighting the cancer cells. Only then can we prompt the body’s response to the tumor and treatment.
Using AI-powered image analysis for patient immune profiling improves our approaches in Precision Oncology:
- It accelerates and de-risks clinical immuno-/oncology development programs
- It supports clinical decision making by uncovering the immune status of the patient’s tumor using multiplex IHC panels
- It enhances patient profiling capabilities by combining tissue biomarker analysis with molecular information
Director Translational and Innovation Management, Definiens
Monika holds a PhD in Biology obtained at the University of Karlsruhe, Germany. Before joining Definiens AG she held various positions in the pharmaceutical industry with a strong record in biochemical science and clinical drug development with a focus on immune oncology.
Her interests are in AI-driven digital pathology and multi-omics approaches to understand the biology of the disease and the mode of action of applied therapies. She is dedicated to developing strategies for predictive and prognostic biomarkers using the information retrieved from the tissue phenome.